Our Institute
Clinical Trials
Our Science
News
International Patients
- International Patients
- International patient Service Care
- Travel Arrangement and Hospital Admission
- FAQ
- Contact Us
Breast Cancer Series I - Personalized 4sCAR-T Immunotherapy
GIMI - Liru Bao
Breast cancer is one of the most common malignant tumors in women, and even men may develop the disease. It originates from the epithelial cells of the breast ducts or lobules, with tumor formation due to the abnormal proliferation of cells. According to pathological and molecular features, breast cancer can be classified into various types, among which invasive ductal carcinoma is the most common, accounting for about 70%-80% of all cases, followed by invasive lobular carcinoma, accounting for 10%-15%. In addition, there are molecular subtypes of breast cancer such as triple-negative carcinoma, HER2-positive carcinoma, and hormone receptor-positive carcinoma. The initial treatment mainly involves surgery, which may be combined with radiotherapy to reduce the risk of metastasis and recurrence, while advanced or metastatic breast cancer requires a combination of chemotherapy, targeted therapy, endocrine therapy and even immunotherapy to control tumor progression and prolong survival.
CAR-T (Chimeric Antigen Receptor T) cell immunotherapy uses genetic engineering techniques to modify the patient's own T-cells to express the CAR gene that specifically recognize tumor surface antigens, thereby precisely kill the tumor cells. The core of this treatment is "target" identification! Antigens that are highly expressed on the surface of breast cancer cells and low in normal tissues need to be identified to ensure specificity and safety. In contrast to the success of CAR-T therapy in hematological cancer (e.g., leukemia), breast cancer, as a solid tumor, faces more obstacles in CAR-T therapy.
In breast cancer, the feasibility of CAR-T therapy is currently in the exploratory phase, with potential but also some limitations.
Short-term: as an addition to the "end-line therapy" in advanced refractory breast cancer (e.g., HER2-positive drug-resistant, triple-negative breast cancer), with potential benefit in rigorously screened patients (e.g., high expression of specific targets, low tumor load).
Long term: need to rely on target optimization, innovative CAR modification and combination strategies to break through solid tumor barriers, and in the future may become a part of comprehensive treatment for some subtypes of breast cancer (e.g., triple-negative, HER2-positive), as well as in combination with existing standard treatments (e.g., surgery, radiotherapy, and targeted agents).
In addition to research in hematological cancer, Shenzhen Geno-Immune Medical Institute (GIMI) has carried out individualized immune cell therapy treating solid tumors. GIMI has conducted numerous clinical trials with partner hospitals to apply an advanced CAR-T therapy strategy for treating breast cancer (NCT04430595 “Multi-4sCAR-T therapy targeting breast cancer”、NCT04429451 “PSMA-specific CAR-T cell therapy、NCT03535246 ”Immunotherapy based on tumor associated antigen-specific immune effector cells”; NCT04432649 Multicenter trial of phase I/II studies on CD276 (B7-H3) positive solid tumors treated with 4sCAR-276). The following cases represent the current progress of GIMI’s multi-4sCAR-T therapy in targeting breast cancer.
Case 1
In 2021, a 70-yr female was diagnosed with stage IIA invasive ductal carcinoma of the right breast, and received right mastectomy with tumor removal. Chemotherapy was initiated with the TCHP regimen, trastuzumab, patuximab combined with docetaxel and carboplatin, along with seven herceptin combination treatments.
In 2023, the patient was evaluated and participated in GIMI-initiated personalized immune cell therapy. Based on the precision immunohistochemistry (IHC) staining, which identified the tumor target antigens, 4sCAR-T cell therapy was planned for GD2/PSMA dual target. Following 4sCAR-T therapy, the patient did not have any physical discomfort or fever, and blood pressure remained normal; on the third day after the CAR-T infusion, her white blood cell count was slightly reduced, and received white blood cell elevation treatment; the indicators quickly improved. After observation and recovery, the patient was discharged from the hospital on the 14th day after CAR-T infusion. After discharge, the follow-up showed improved physical recovery.
On subsequent follow-ups, the patient indicated that she recovered exceptionally well with improved appetite and regained 3 kilograms of body weight. In several subsequent physical checkups, all the indexes remained stable free of any abnormality. The patient has been followed up for more than 2 years with overall satisfactory condition.
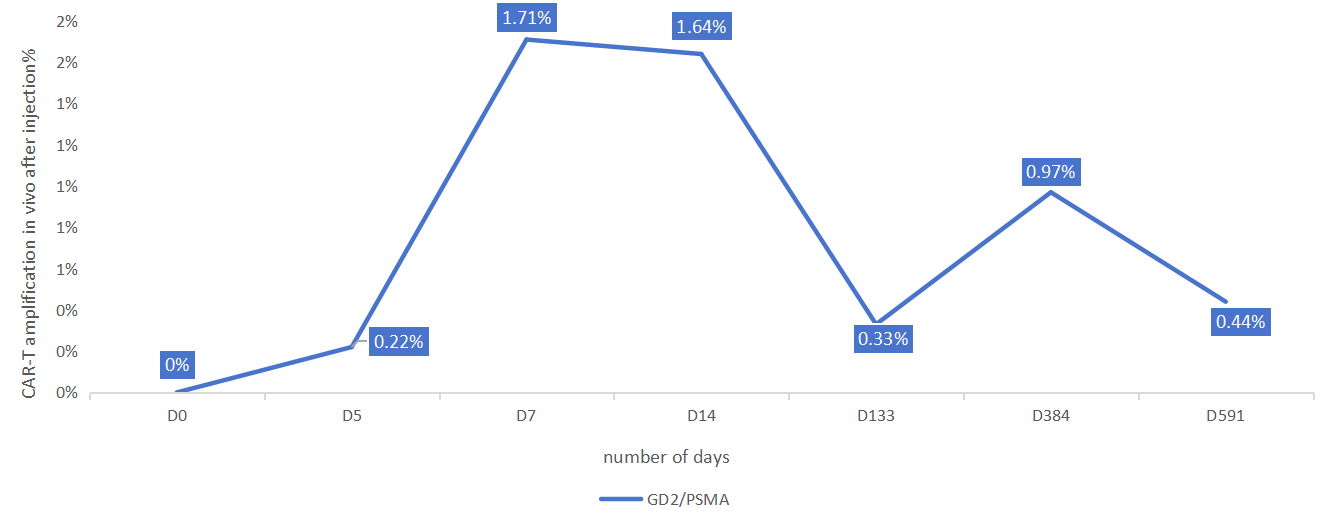
Figure 1. The kinetics of 4sCAR-GD2/PSMA dual-target CAR-Ts in vivo in 591 days
Case 2
A 54-yr-old patient underwent CT examination of the chest and abdomen in 2023, and discovered that the density of the right breast was higher than nearby normal tissues, showing signs of slight enlargement. The examination was consistent with breast cancer, with involvement of lymph nodes in the axillae bilaterally, and further, with scattered small nodes in the lungs bilaterally. Thus, metastatic tumors were considered, partly inflammatory, accompanied by fibrosis foci in the lungs, with a tendency to increase in size. The patient underwent treatment with targeted drugs and chemotherapy.
After a combination of targeted agents and chemotherapy, the patient was enrolled in the GIMI sponsored multi-4sCAR-T therapy. The CAR-T regimen was customized based on precision IHC staining results, which identified PSMA and Mesothelin as target antigens. After the infusion of 4sCAR-T cells aimed at both of the above targets, the patient did not experience any physical discomfort, had normal appetite and could rest as usual. For consolidation, the patient received additional CD276 (B7-H3) 4sCAR-T infusion. Shortly after CAR-T infusion, the patient felt slight pain in the middle of the right breast, but the original peripheral pain disappeared, and the overall scope of the pain had diminished. In a few days, the pain had significantly reduced and then disappeared, and the breast area felt soft to touch, and no feeling of pain when pressed. Follow-up examinations showed gradual improvement in the breast, and subsequent re-examinations showed that the outline of the lump had disappeared, and further examinations confirmed marked improvement. The patient's overall condition has improved since CAR-T therapy, especially the discomfort in the breast area has largely diminished.
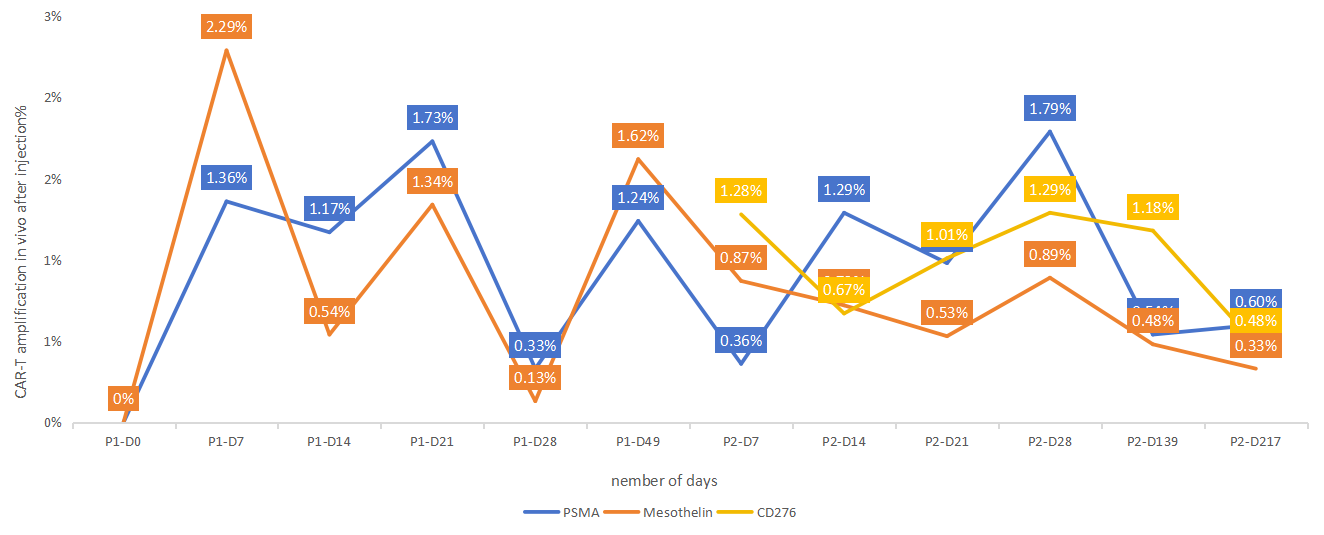
Figure 2. Kinetics of simultaneous amplification of multiple CAR-Ts in vivo
These cases exemplify the advanced individualized precision-targeted 4sCAR-T therapy technology, which has demonstrated great potential in breast cancer treatment, and provides a reference for subsequent research and applications.
Conclusion:
The treatment for breast cancer mostly adopts comprehensive programs such as surgery, chemotherapy, radiotherapy, endocrine therapy, and targeted therapy, etc. The CAR-T immunotherapy, by modifying T cells to make them precisely kill the tumor cells, is still in the exploratory stage but may become a major complementary treatment tool for advanced refractory breast cancer. The above cases demonstrate that the individualized 4sCAR-T multi-target combination therapy (targeting GD2/PSMA, PSMA/Mesothelin, and CD276) after conventional surgery, chemotherapy, and targeted therapy, can obtain beneficial outcomes, and importantly, the 4sCAR-T regimens are safe with little to no adverse effects. Both patients recovered well from the multi-CAR-T infusions and the disease symptoms have largely disappeared, with significantly improved conditions. As compared with radiation and chemotherapies, the 4sCAR-T therapy is not associated with drug-related toxicity, and both patients have recovered well and regained good quality of life without disease recurrence.