Our Institute
Clinical Trials
Our Science
News
International Patients
- International Patients
- International patient Service Care
- Travel Arrangement and Hospital Admission
- FAQ
- Contact Us
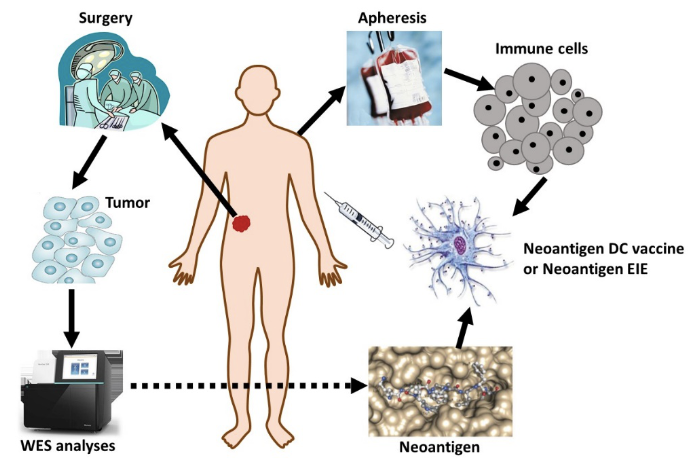
Compared to normal somatic cells, gene mutations which lead to uncontrolled proliferation are always found in the cancer cells. The mutations are associated with specific amino acid sequence changes. These variant proteins are hydrolyzed to small peptides by intracellular proteases, and presented to the cell surface (through MHC), and then activate a cancer immune response after recognized by T cells. These mutation fragments are called tumor "neoantigen”(nAg).
To identify tumor neoantigens for immunotherapy, it is necessary to obtain DNA and RNA from the tumor specimens as well as normal blood cells for comparison. The samples are sent to the gene sequencing and nAg analysis company through GIMI for total exon sequencing analysis. The nAg analysis task takes about 4-6 weeks. These nAgs once predicted by sequencing analysis will then be commercially synthesized as individualized neopeptides for immunotherapy use.
Based on the neopeptides identified by sequence modeling, we will prepare DC vaccine (nAg-DCv) and nAg-specific T cells (nAg-EIE) to treat the cancer. These novel tools offer an individualized targeted immunotherapy to treat the cancer, which is highly specific with little to no toxicity. It takes 7 days and 22 days for nAg-DCv and nAg-EIE preparation, respectively. Neoantigen Immunotherapy has the advantages of high specificity, low to no toxicity, individualized tumor targeting with prolonged immune memory. GIMI has registered trials for tumor nAg immunostherapy (NCT03914768, NCT04085159).