Our Institute
Clinical Trials
Our Science
News
International Patients
- International Patients
- International patient Service Care
- Travel Arrangement and Hospital Admission
- FAQ
- Contact Us
Breakthrough Against the "King of Childhood Cancer" - Serial 4sCAR-GD2 T Cell Infusions Significantly Improve Overall Survival of High-Risk Neuroblastoma Patients
GIMI - Rui Zhang
Neuroblastoma (NB), often coined as "King of Childhood Cancer", portends a particularly dismal prognosis for high-risk pediatric patients. Recent advances in GD2 antigen-targeted chimeric antigen receptor T-cell (CAR-T) therapy have emerged as a promising therapeutic avenue.
Collaborative research spearheaded by Shenzhen Geno-immune Medical Institute (GIMI) has pioneered the development of an innovative fourth generation GD2-specific CAR-T technology, incorporating an intrinsic safety mechanism (4sCAR-GD2), which has been granted both U.S. and Chinese patents (US20210309757A1; CN108948211B).
Critical analysis of recent clinical data reveals that a strategy employing serial infusions of this 4sCAR-GD2 T cell product significantly improves survival and disease control, offering renewed hope for pediatric patients with high-risk NB.
I. The challenge of high-risk NB
NB represents the most prevalent extracranial solid tumor in the pediatric population, predominantly affecting children under 5 years of age [1]. Approximately 70% of NB patients present with widely metastatic disease (frequently involving bone marrow and bones) at diagnosis, classifying as high-risk. Despite iterative improvements in multimodal therapy, including dose-intensive chemotherapy, surgical resection, radiotherapy, autologous stem cell transplantation, and GD2-targeted monoclonal antibodies, long-term outcomes remain suboptimal. The 5-year survival rate for high-risk patients remains around 50%. Relapsed or refractory disease continues to pose a significant therapeutic challenge [2].
II. The GD2 CAR-T - a new precision weapon
Disialoganglioside GD2 demonstrates consistent, high expression on NB cell surface, thus becoming a compelling therapeutic target. The GD2-directed CAR-T therapy involves genetic modification of autologous T lymphocytes to confer specific recognition and cytotoxic activity against GD2-positive tumor cells. This approach constitutes a promising immunotherapeutic strategy for relapsed/refractory NB [3,4].
Leveraging extensive translational medicine expertise in cellular immunotherapy, GIMI has actively collaborated with several hospitals through investigator-initiated trials (NCT02992210 -"Multicenter phase I/II dtudy of 4SCAR-GD2 in GD2-positive solid tumors"; NCT04637503 - "4SCAR-T therapy targeting GD2, PSMA and CD276 for neuroblastoma"). Some key clinical findings are presented below.
III. Pivotal clinical data: superiority of the serial 4sCAR-GD2 strategy
A retrospective analysis of 87 NB patients receiving 4sCAR-GD2 T cell therapy has accumulated compelling clinical insights.
1. Patient characteristics: The enrolled patients were in a broad age distribution (range 2-28 years; median 5-6 years), with >60% under stage IV high-risk conditions, representing a clinically challenging population. Thirty-one patients (n=31) received a single 4sCAR-GD2 T cell infusion, while 56 patients (n=56) underwent serial 4sCAR-GD2 infusions. The infused cell dose ranged from 0.32 to 10.39 × 10⁶ cells/kg (median 2.5 × 10⁶ cells/kg).
2. Comparative efficacy (primary endpoints):
Table 1. Comparative efficacy and survival outcomes - single vs. serial 4sCAR-GD2 T cell infusion cohorts
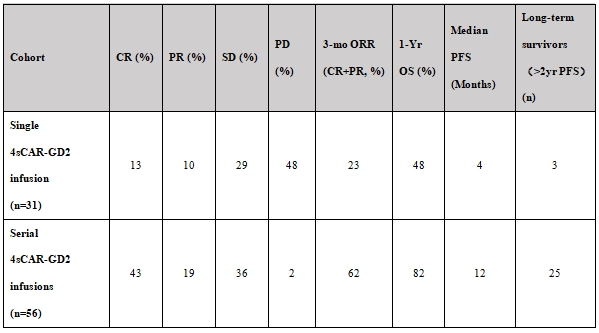
Key findings from Table 1:
· Single infusion cohort (n=31): Demonstrated a modest 3-month objective response rate (ORR) of 23% (CR: 13%; PR: 10%) and disease control rate (DCR = CR+PR+SD) of 52%. Notably, 48% exhibited progressive disease (PD) within 3 months, indicating poor overall prognosis.
· Serial infusion cohort (n=56): Achieved a significantly higher 3-month ORR of 62% (CR: 43%; PR: 19%) and a remarkable DCR of 98% (SD: 36%; PD: 2%). The therapeutic efficacy was substantially superior to the single infusion cohort.
3. Survival benefit:
· Progression-Free Survival (PFS): The serial infusion cohort exhibited a median PFS of 12 months, significantly exceeding the 4-month median PFS observed in the single infusion cohort (p<0.001).
· Overall Survival (OS): The 1-year OS rate for the serial infusion cohort was 82%. Critically, among high-risk patients with bone marrow involvement, 1-year OS was 82% in the serial group versus 48% in the single infusion group.
· Long-term Response: Within the serial infusion cohort, 44.6% (25/56) maintained progression-free status beyond 2 years. The longest follow-up confirmed sustained remission, with the longest ongoing complete response (CR) or partial response (PR) documented at 53 months (4.4 years) and 67 months (5.6 years) post-initiation of therapy, demonstrating profound disease control potential.
4. Relapse Management: For patients experiencing subsequent relapse, repeated 4sCAR-GD2 infusions or combinatorial regimens incorporating chemotherapy/radiotherapy yielded secondary remissions in some cases, thus expanding the salvage treatment options.
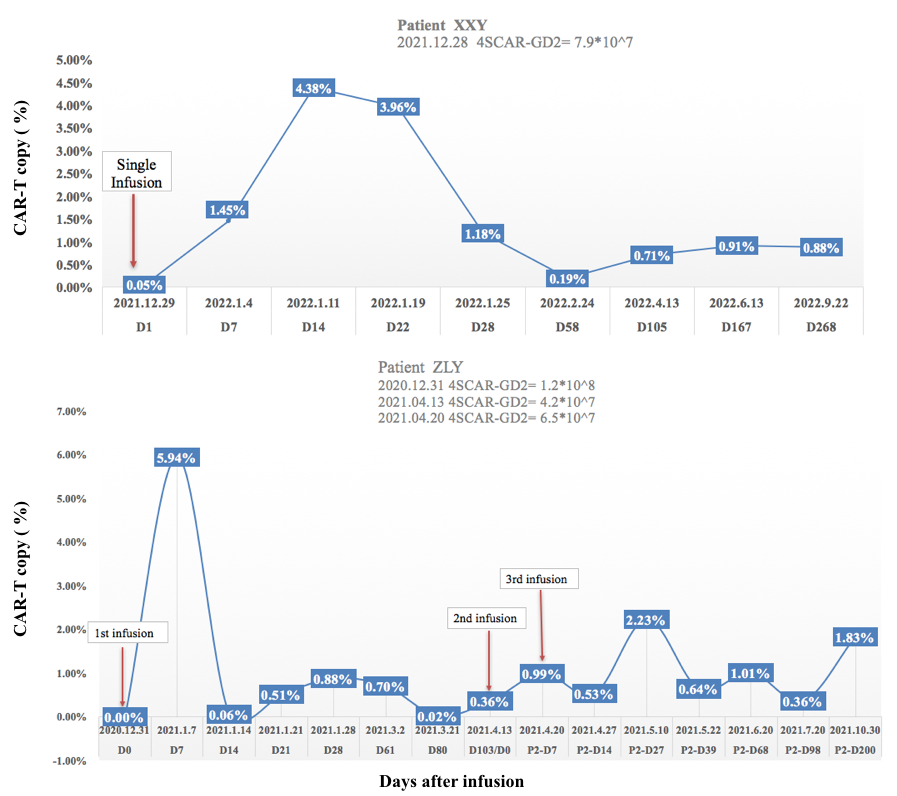
Figure 1. Kinetics of 4sCAR-GD2 expansion in blood with a single versus serial CAR-T infusions
Figure 1 illustrates that the 4sCAR-GD2 T cell persistence at >0.5% in peripheral blood following scheduled booster infusions is correlated with sustained disease control and long-term remission.
IV. Safety profile: manageable toxicity without increased risk in serial dosing
Table 2. Adverse event profile following the 4sCAR-GD2 therapy
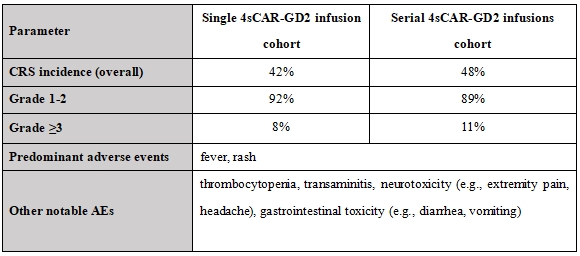
Safety assessment from Table 2:
· Cytokine Release Syndrome (CRS) occurred in approximately 45% of all treated patients, predominantly low-grade (grade 1-2, characterized by fever and rash). Higher-grade CRS (≥ grade 3) was infrequent.
· Critically, serial infusions did not significantly elevate the incidence or severity of CRS compared to single infusion (p=0.42).
· Other manageable adverse events included hematologic toxicity (thrombocytopenia), hepatic transaminase elevations, neurotoxicity, and gastrointestinal disturbances.
V. Clinical implications and future considerations
1. Serial infusion paradigm: Robust clinical evidence mandates the adoption of serial 4sCAR-GD2 infusions as a cornerstone strategy for high-risk NB, particularly stage IV disease with bone marrow involvement, to maximize ORR, DCR, and long-term PFS/OS.
2. Combinatorial approaches: Future clinical investigation should prioritize:
· Evaluating the necessity for consolidation with autologous hematopoietic stem cell transplantation (auto-HSCT) after the CAR-T treatment.
· Rational combination strategies with molecularly targeted agents (e.g., ALK inhibitors) or complementary immunotherapies (e.g., anti-GD2 mAbs, immune checkpoint blockade).
· Assessing the efficacy of dual-target CAR-T strategies in larger prospective trials.
3. Patient stratification: Identifying subsets potentially deriving benefit from optimized single-infusion protocols (e.g., low tumor burden) warrants further study.
4. Personalized salvage therapy: Tailoring therapeutic interventions upon relapse, including CAR-T re-infusion or combinatorial salvage regimens, to increase the potential for sustained disease control.
Conclusion:
GD2-targeted 4sCAR-T therapy represents a transformative advance in the management of high-risk NB. This analysis definitively establishes the critical role of the serial infusion protocols in achieving superior and durable disease control, significantly improving survival metrics within an acceptable safety framework. Continued clinical refinement, exploration of synergistic combination strategies, and development of next-generation constructs (e.g., dual-target CARs) hold immense promise for further improving outcomes against this formidable pediatric malignancy, ultimately offering the potential for cure and extended high-quality survivorship.
References:
1. Sherief, L. M. et al. Tissue factor expression predicts outcome in children with neuroblastoma: a retrospective study. Oncol. Lett. 18, 6347-6354 (2019).
2. Mallepalli, S., Gupta, M. K. & Vadde, R. Neuroblastoma: an updated review on biology and treatment. Curr. Drug Metab. 20, 1014-1022 (2019).
3. Xu, X. et al. 4SCAR-GD2-modified T-cell therapy in neuroblastoma with MYCN amplification: a case report with over 4- year follow-up data. Pediatr. Investig. 4, 55-58 (2020).
4. Yu, L. et al. GD2-specific chimeric antigen receptor-modified T cells for the treatment of refractory and/or recurrent neuroblastoma in pediatric patients. J. Cancer Res. Clin. Oncol. 148, 2643-2652 (2022).