Our Institute
Clinical Trials
Our Science
News
International Patients
- International Patients
- International patient Service Care
- Travel Arrangement and Hospital Admission
- FAQ
- Contact Us
Overcoming the Challenge of Refractory/Relapsed Multiple Myeloma
(MM)- The "King of Blood Cancer"
- Individualized Multi-Target 4sCAR-T Therapy Brings New Hope
By: GIMI - Rui Zhang
Introduction: understanding the "king of blood cancer" - multiple myeloma
Let's discuss a disease known as multiple myeloma (MM). This is a malignant tumor originating from plasma cells (a type of immune cell) within the bone marrow. It insidiously causes these plasma cells to proliferate uncontrollably, producing large quantities of "bad" proteins (monoclonal immunoglobulins) that damage the body, leading to problems such as anemia, bone pain, and kidney damage [1,2].
Although the advent of novel drugs (immunomodulatory agents, proteasome inhibitors) has significantly extended patient’s survival (median survival has nearly doubled in the last decade), unfortunately, many patients ultimately develop resistance to existing therapies, experience disease relapse and face therapeutic dilemmas [3,4]. Especially for younger patients, there is a strong desire to find approaches to achieve long-term remission or even a cure.
New weapons: immunotherapy ignites hope
Faced with this challenge, scientists have turned their attention to the body's own "anti-cancer warriors" - immune cells. Immunotherapy has thus emerged as a new beacon of hope against multiple myeloma. One revolutionary technology is CAR-T cell therapy.
Currently, CAR-T therapies targeting different "markers" have achieved remarkable results in treating other blood cancers (e.g., lymphoma, leukemia). So, which "markers" are most effective against multiple myeloma? Researchers have identified some highly promising targets including BCMA (highly expressed on myeloma cells) [5,6], CD38 (widely present) [7,8], CD138Mul (associated with plasma cell development) [9], and GPRC5D (specifically and highly expressed on myeloma cells, independent of BCMA) [10-12].
Leveraging deep expertise in cellular immunotherapy, Shenzhen Geno-immune Medical Institute (GIMI) has actively collaborated with many hospitals to develop personalized multi-target CAR-T therapy for treating multiple myeloma (NCT03271632 – “Multi-CAR T cell therapy in the treatment of multiple myeloma”; NCT06429150 – “Frontline combination CAR- T cell therapy for multiple myeloma or plasmacytoma”, NCT06435910 - “Engineered dendritic cell vaccines for multiple myeloma”). A few case examples of these innovative studies are presented here.
Precision strike: GIMI’s personalized multi-target CAR-T "combo punch"
This section highlights an advanced, personalized strategy: multi-target combination CAR-T therapy (4SCAR2.0). Its core principle is tailoring a unique "combo punch" CAR-T regimen for each patient based on the unique combination of "markers" identified on the myeloma cell surface, employing multiple different CAR-T cells simultaneously in a coordinated attack!
Below are the treatment details of three multiple myeloma patients who received this personalized "combo punch" CAR-T therapy, all achieved excellent outcomes:
1. Patient K (41 yrs): Presented with severe disease at diagnosis, including widespread lytic bone lesions and pathological fractures. After receiving a CAR-T combination targeting BCMA and CD19, the levels of disease-related "bad" proteins in the body continuously decreased, achieving a stringent complete response (sCR) within 4 weeks! A subsequent booster infusion of GPRC5D CAR-T was administered. The patient has maintained in remission for over 33 months. The only side effects observed was mild and transient fever (cytokine release syndrome - CRS grade 0-1).
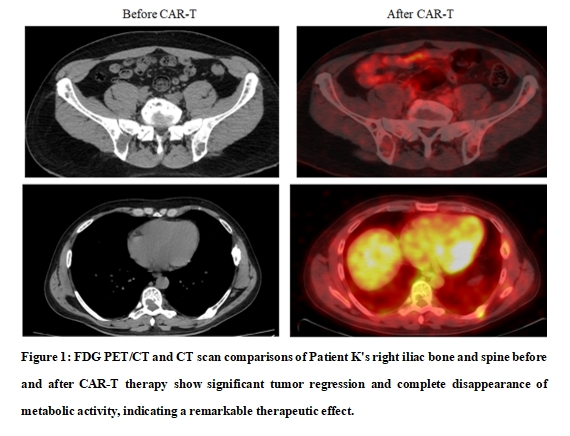
2. Patient A (63 yrs): Relapsed after multiple therapies, including two autologous stem cell transplantations, and progressed to more aggressive plasma cell leukemia. A CAR-T "combo punch" was given to target BCMA, CD19, and CD317. After the treatment, abnormal indicators in blood and urine started to decline steadily, achieving sCR within 4 months. Following a booster infusion of GPRC5D CAR-T, a stable remission has sustained for 30 months. Again, the CAR-T treatment course was very smooth, with no significant side effects.
3. Patient V (48 yrs): Diagnosed with severe bone destruction and fractures. The treatment plan was the most complex of the three: starting with BCMA single-target CAR-T, followed by dual-target CAR-Ts (simultaneously targeting CD138 and BCMA, plus another targeting CD19 and CD22). The treatment was accompanied by transient fever, headache, and bone pain (mild CRS, grade 1-2), and the disease indicators showed continuous improvement. The patient achieved sCR in 4 months, and remission has sustained for more than 30 months.
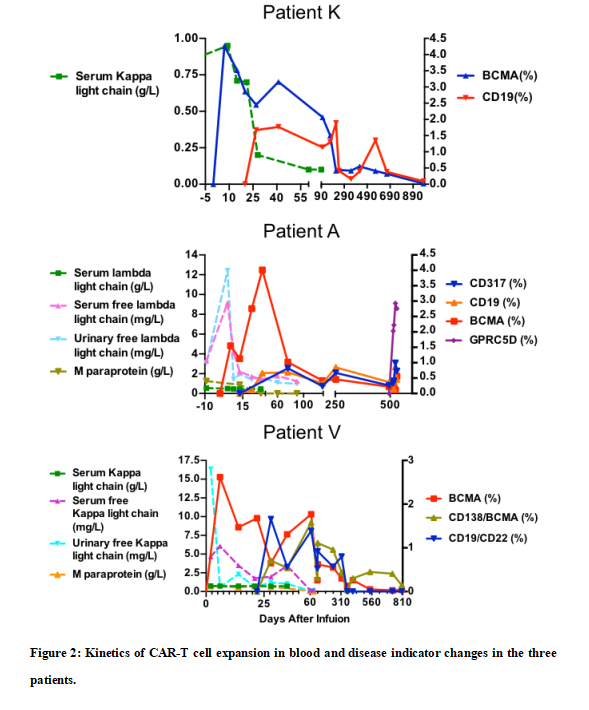
Figure 2 demonstrates that the multiple CAR-T cells successfully expanded and persisted long-term in all three patients, with disease indicators subsequently declined back to normal ranges. This CAR-T and disease indicator dynamic closely associated with excellent therapeutic outcomes overcoming potential relapse.
Why multi-target CAR-T strategy is superior?
• Precision targeting prevents antigen escape: Targeting only one marker allows cunning cancer cells to potentially "hide" or "shed" that antigen (antigen loss) to evade immune attack. Using CAR-T cells targeting multiple tumor antigens simultaneously creates a "combination punch," significantly reducing cancer cells' escape potential.
• Personalized target selection: Each patient's cancer cell "antigen profile" may differ. By examining these antigens based on precision immunostaining (e.g., via immunohistochemistry/flow cytometry screening), the optimal CAR-T combination can be planned for each patient, achieving truly a "bespoke regimen" therapy.
• Robust and sustained remission: Studies show that multi-CAR-T combination strategies exhibit robust expansion and long-term persistence in patients, enabling sustained control or even elimination of residual cancer cells.
The success of this study is based on several key factors/strategies:
1. Properly laying the groundwork (bridging therapy): while planning for CAR-T cell therapy, patients received chemotherapy, targeted drugs, or even autologous stem cell transplant as "bridging therapy" to effectively control tumor burden, preventing rapid disease progression before the CAR-T treatment.
2. Optimal timing: administering CAR-T infusion when patients are in a relatively stable condition (not in rapid progression), which helps reduce severe side effects, allowing CAR-T cells to "establish a foothold" and "expand their forces" in a more favorable in vivo environment.
3. Good T-cell quality: early collection and cryopreservation of a patient’s T-cells (during initial disease stages or early treatment) preserves better, more vigorous T-cells for future CAR-T manufacturing.
Conclusion: A promising innovative treatment paving the path to rebirth
These three successful cases demonstrate the immense potential of personalized, multi-target CAR-T therapy (4SCAR2.0) for treating multiple myeloma, especially in patients with relapsed/refractory disease after multiple prior therapies. The treatment approach has been proven not only safe and feasible, but also may achieve a robust and sustained remission.
Moving beyond merely extending survival to pursuing deeper, longer-lasting remissions and even functional cures, the next-generation immunotherapies like multi-4sCAR-T are revolutionizing the fight against multiple myeloma - the "king of blood cancer." As research advances and treatment strategies become optimized, this "combo punch" 4sCAR-T approach gives high hope to benefit more patients and illuminating a path to rebirth!
References:
[1] Kumar SK, et al. Multiple myeloma. Nat Rev Dis Primers. 2017; 3:17046.
[2] Braggio E, Kortüm KM, Stewart AK. Snapshot: Multiple Myeloma. Cancer Cell. 2015 Nov 9;28(5):678-678.e1. doi: 10.1016/j.ccell.2015.10.014. PMID: 26555176; PMCID: PMC4864595.
[3] Kumar, Shaji K et al. “Improved survival in multiple myeloma and the impact of novel therapies.” Blood vol. 111, 5(2008): 2516-20. doi:10.1182/blood-2007-10-116129.
[4] Seckinger A, Delgado JA, Moser S, et al. Target Expression, Generation, Preclinical Activity, and Pharmacokinetics of the BCMA-T Cell Bispecific Antibody EM801 for Multiple Myeloma Treatment. Cancer Cell. 2017;31(3):396-410. doi:10.1016/j.c cell.2017.02.002
[5] Yu B, Jiang T, Liu D. BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol. 2020 Sep 17;13(1):125. doi: 10.1186/s13045-020-00962-7. PMID: 32943087; PMCID: PMC7499842.
[6] Seckinger A, Delgado JA, Moser S, et al. Target Expression, Generation, Preclinical Activity, and Pharmacokinetics of the BCMA-T Cell Bispecific Antibody EM801 for Multiple Myeloma Treatment. Cancer Cell. 2017;31(3):396-410. doi:10.1016/j.c cell.2017.02.002
[7] Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N Engl J Med. 2015;373(13):1207-1219. doi:10.1056/NEJMoa1506348
[8] Deckert J, Wetzel MC, Bartle LM, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res. 2014;20(17):4574-4583. doi:10.1158/1078-0432. CCR-14-0695
[9] Guo B, Chen MX, Han QW, Hui F, Dai HR, Zhang WY, Zhang YJ, Wang Y, Zhu HL, Han WD, CD138-directed adoptive immunotherapy of chimeric antigen receptor (CAR)-modified T cells for multiple myeloma, Journal of Cellular Immunotherapy, Volume 2, Issue 1, 2016, Pages 28-35, ISSN 2352-1775, https://doi.org/10.1016/j.jocit.2014.11.001.
[10] Mailankody S, Devlin SM, Landa J, Nath K, et al. GPRC5D-Targeted CAR T Cells for Myeloma. N Engl J Med. 2022 September 29; 387(13): 1196–1206. doi:10.1056/NEJMoa2209900.
[11] Xia J, et al. Anti-GPRC5D CAR T-cell therapy as a salvage treatment in patients with progressive multiple myeloma after anti-BCMA CAR T-cell therapy: a single-centre, single-arm, phase 2 trial. Lancet Haematol. 2025 May;12(5): e365-e375.
[12] Yang X, Wang F, Yuan X, Yang B, Chen J, Cheng J, Liu G, Tang D, Xu X, Wang S, He Z, Liu Y, Li Y. Efficacy and safety of chimeric antigen receptor T cells targeting BCMA and GPRC5D in relapsed or refractory multiple myeloma. Front Immunol. 2024 Dec 23:15:1466443.