Our Institute
Clinical Trials
Our Science
News
International Patients
- International Patients
- International patient Service Care
- Travel Arrangement and Hospital Admission
- FAQ
- Contact Us
Gene Therapy Brings Hope to Adrenoleukodystrophy (ALD) Patients
Author: GIMI - Yingying WANG
ALD - A Silent Crisis Behind a Rare Disease
Adrenoleukodystrophy (ALD) is a rare X-linked recessive genetic disorder that predominantly affects males. The disease stems from mutations in the ABCD1 gene. Under normal circumstances, ABCD1 is widely expressed in cells such as neurons in the brain, adrenal cortical cells, and peripheral blood monocytes, playing a critical role in maintaining the metabolic balance of very long-chain fatty acids (VLCFAs) in cells [1, 2].
Studies have shown that the expression of the ABCD1 gene varies significantly among different cell types. In adrenal cortical cells, the expression level of ABCD1 is relatively high, which is closely related to its efficient metabolism of VLCFAs to maintain normal adrenal cortical hormone synthesis [2]. Mutations in ABCD1 disrupt protein function, leading to VLCFA accumulation in the brain and adrenal glands. This not only causes progressive damage to the nervous system, manifesting as symptoms like vision loss, hearing impairment, movement disorders, and cognitive decline, but also results in adrenal cortical insufficiency, seriously affecting the quality of life and survival prognosis. Although the number of patients with such rare diseases is relatively small, the physical and mental suffering they endure cannot be ignored.
Limitations of Traditional Treatments
Currently, traditional treatment options for ALD patients are limited, with symptomatic treatment being the core strategy. For example, drugs are used to relieve neurological symptoms, and hormones are supplemented to maintain adrenal function [3]. However, these treatments can only alleviate symptoms and cannot fundamentally solve the disease caused by the genetic defect. Patients have lifelong dependence on drug therapy, which not only severely affects the quality of life but also leads to gradual deterioration of health condition. Long-term drug treatment may also cause a series of adverse reactions, such as liver and kidney damage and decreased immunity, further increasing the patients' physical burden and mental stress.
Hematopoietic stem cell transplantation (HSCT) is an advanced treatment that has been given in clinical practice. It reconstitutes the patient's hematopoietic and immune systems by transplanting a healthy donor’s hematopoietic stem cells into the patient's body. Clinical studies have shown that for early-stage ALD patients with mild conditions, HSCT can delay the progression of neurological damage and even improve symptoms in some cases, but this approach faces many limitations [4, 5]. Firstly, it only benefits patients diagnosed very early without or with very mild symptoms; secondly, it is difficult to find matched donors, resulting in low success rates; and lastly, the transplantation risk is high, and patients need long-term use of immunosuppressants to prevent rejection, which often increases the risk of complications such as infections; in addition, the high cost of transplantation limits its wide application.
Novel Gene Therapy: opening a new door to cure
As a revolutionary treatment strategy, gene therapy has brought unprecedented hope for a cure to ALD patients. Its core principle is to use advanced technology to introduce the normal ABCD1 gene into the patient's body, replacing or repairing the abnormal ABCD1 gene, thereby fundamentally correcting the disease state [6]. Relying on its profound translational experiences in the field of gene therapy, Shenzhen Geno-immune Medical Institute (GIMI) has actively participated in innovative ALD gene therapy research in collaboration with partner hospitals (NCT03727555, NCT03725670) [7, 8].
Here some treatment cases are introduced:
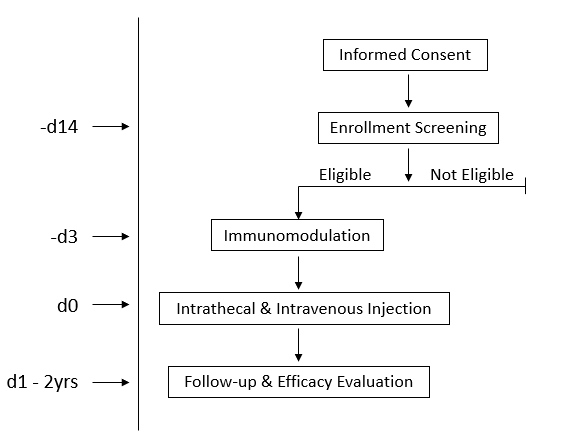
Case 1:
Patient Information: Male, diagnosed at age 10 with ABCD1 exon 1 mutation (c.521A>C, p.Tyr174Cys), ineligible for HSCT, enrolled at age 14 (ALD Score I-II, NFS 3).
Gene Therapy: Methylprednisolone was used for immunosuppressive conditioning to avoid transgene rejection. On Day 0, the ABCD1 lentivector was administered via lumbar puncture for intrathecal injection. Methylprednisolone was continued for immune regulation, with the dosage gradually reduced, and discontinued after 1 month.
Efficacy Evaluation: MRI assessment showed that the patient's disease was stable without progression for one year after gene therapy.
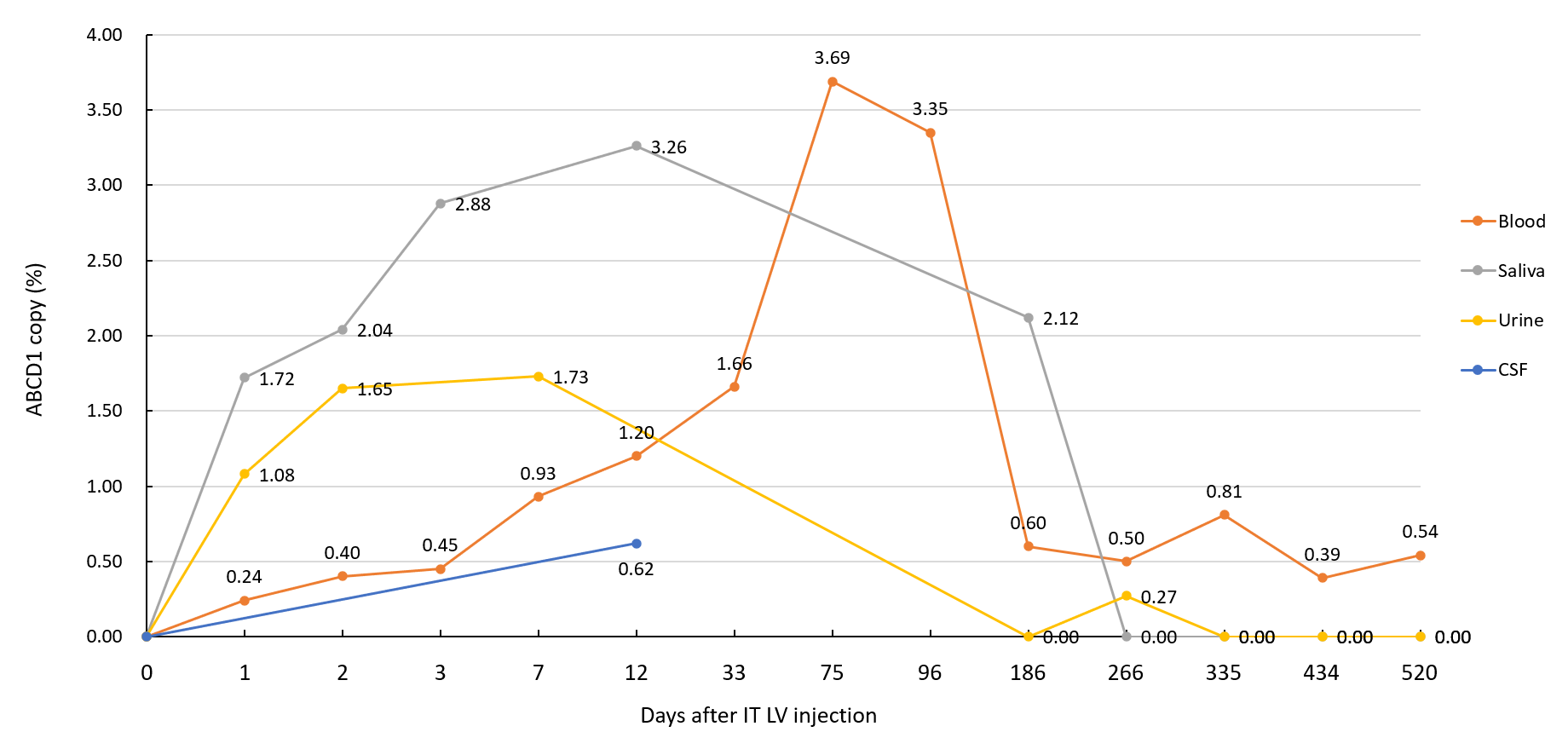
Case 1: Detection of the injected ABCD1 lentiviral gene in the patient
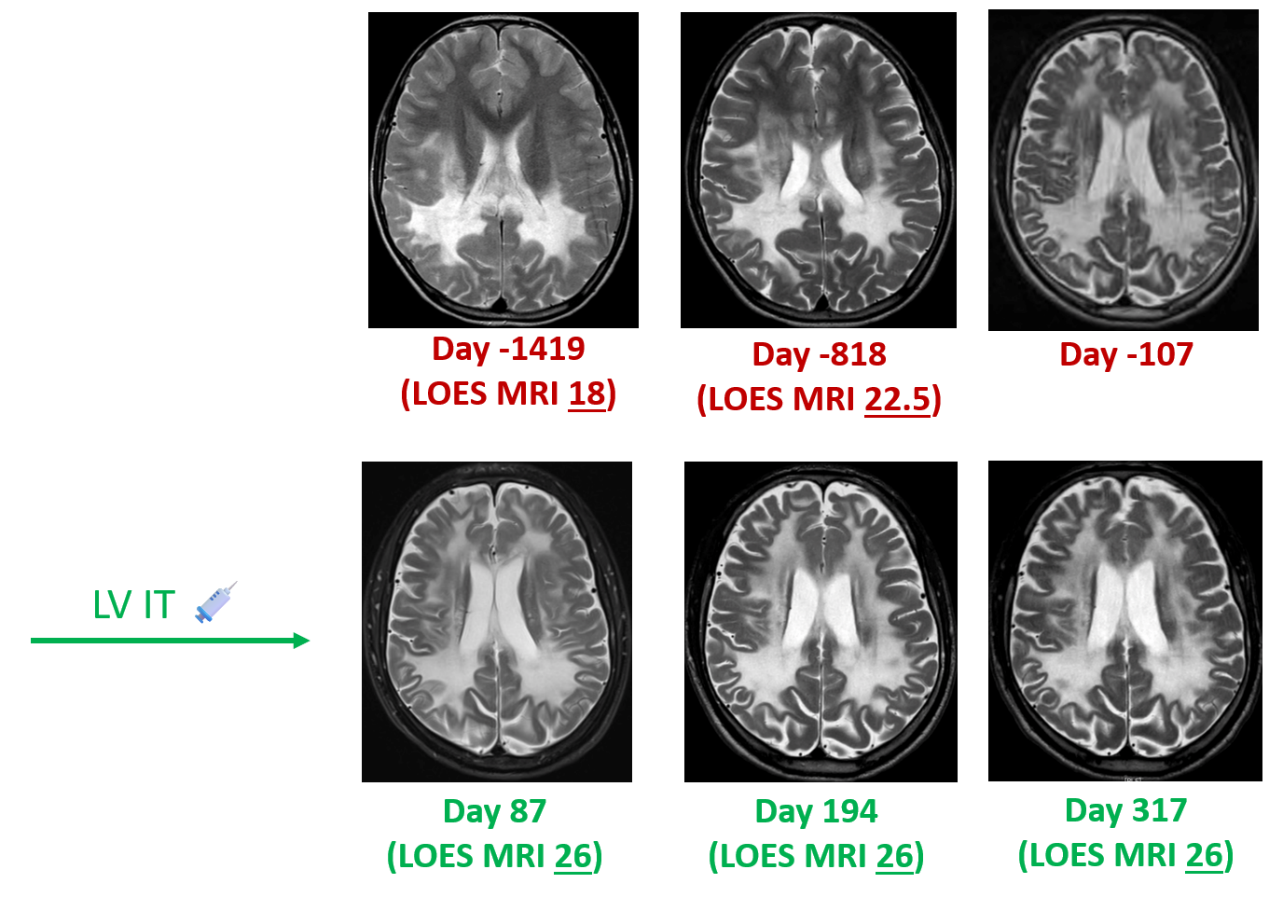
Case 1: Kinetic changes in MRI and LOES scores
Case 2:
Patient Information: Male, diagnosed at age 10 with ABCD1 exon 2 mutation (c.956T>C, p.Leu319Pro), enrolled at age 10 (ALD Score II, NFS 4) with coordination deficits, speech impairment, and vision loss.
First Gene Therapy: Methylprednisolone was used for immunosuppressive regulation. On Day 0, the ABCD1 vector was administered via lumbar puncture for intrathecal injection. Methylprednisolone was continued for immune regulation, with the dosage gradually reduced, and discontinued after approximately 1 month.
Second Gene Therapy: Methylprednisolone was used for immunosuppressive regulation. On Day 171, the ABCD1 lentivector was administered via lumbar puncture for intrathecal injection combined with intravenous injection. Methylprednisolone was continued for regulation, with the dosage gradually reduced, and discontinued after approximately 1 month.
Efficacy Evaluation: MRI assessment on Day 75 after the first treatment showed no disease progression compared with Day -47. MRI assessment on Day 155 showed a vague enlargement of the pathological area; the patient's condition was basically stable behaviorally but developed visual impairment. MRI assessment on Day 268 after the second treatment showed relative stability.
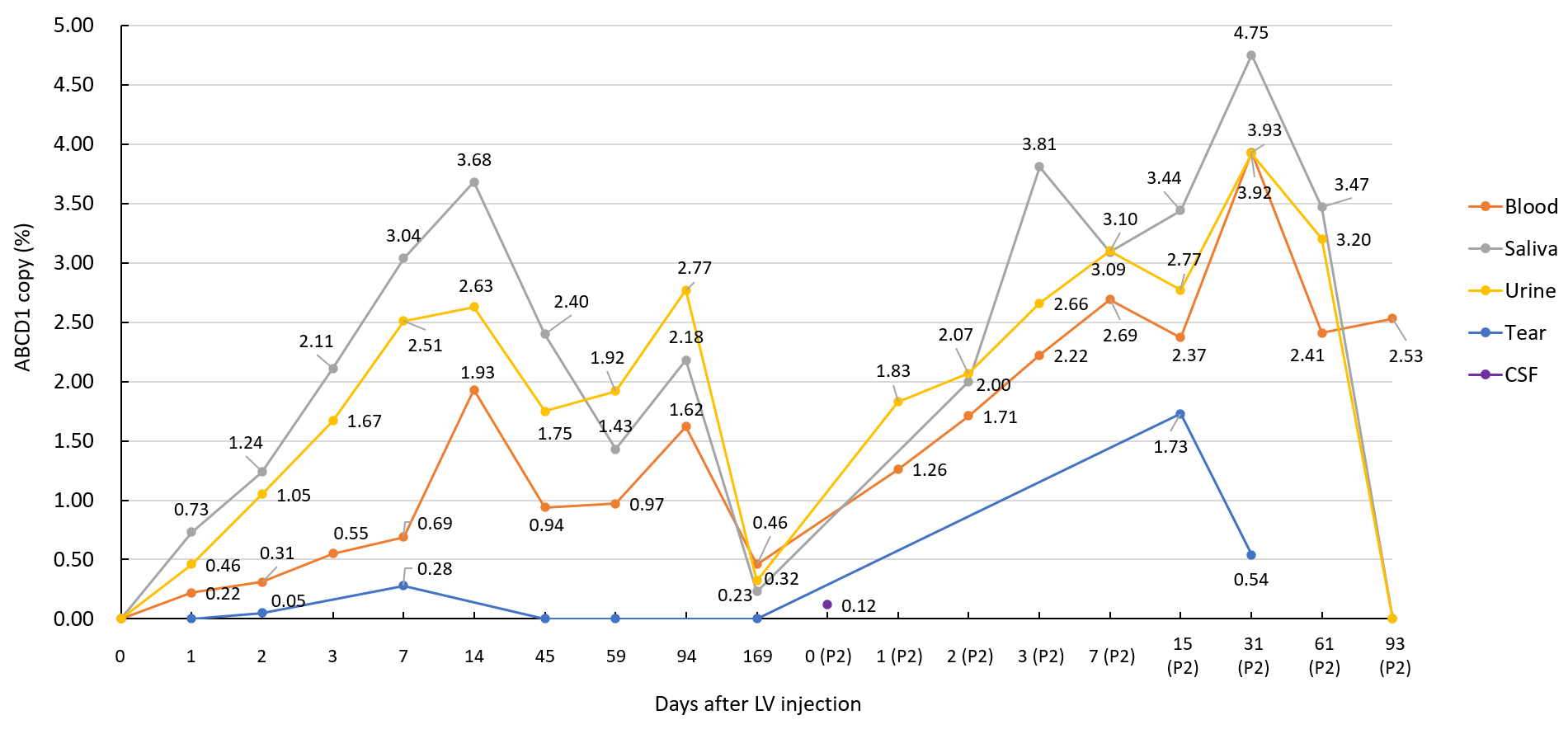
Case 2: Detection of the lentiviral ABCD1 therapeutic gene in the body
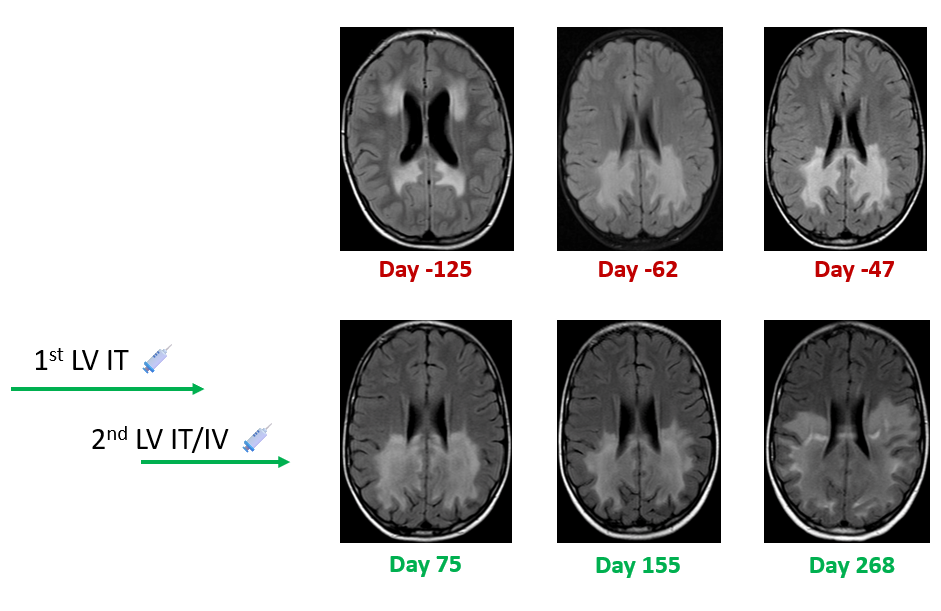
Case 2: Kinetic changes in MRI
Conclusion
The innovative gene therapy for ALD developed by GIMI adopts advanced lentivector technology and a precise and simple injection procedure to ensure the safety and feasibility of the treatment for even symptomatic patients. This novel strategy has established a comprehensive follow-up and evaluation system, enabling continued tracking of treatment effects and adjustment of subsequent plans. The study has finished its phase I clinical trial and is entering phase II stage. It is firmly believed that with the continuous advancement of technology, more ALD patients will benefit from the novel treatment and obtain an improved quality of life.
References
1. Engelen M, Kemp S, Poll-The B T. X-Linked Adrenoleukodystrophy: Pathogenesis and Treatment[J]. Current Neurology & Neuroscience Reports, 2014, 14(10):486.
2. Ohashi T. Gene therapy for lysosomal storage diseases and peroxisomal diseases[J]. Nature Publishing Group, 2019,64(2):139-143.
3. Kemp S, Berger J, Aubourg P. X-linked adrenoleukodystrophy: Clinical, metabolic, genetic and pathophysiological aspects[J]. Biochimica Et Biophysica Acta, 2012, 1822(9):1465-1474.
4. Eichler F, Duncan C, Musolino P L, et al. Hematopoietic Stem-Cell Gene Therapy for Cerebral Adrenoleukodystrophy[J]. N Engl J Med, 2017, 377(17):1630-1638.
5. Ciftciler R, Goker H, Buyukask Y, et al. The experience of allogeneic hematopoietic stem cell transplantation in a patient with X-linked adrenoleukodystrophy[J]. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis, 2020, 59(1):102583.
6. LIU Yun-yun, CHUNG Tsai-Hua, CHANG Lung-Ji. School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan 610000, China.
7. Gong J, Liu Y, Chung T H, et al. gene therapy in an early disease onset ALD mouse model[J]. Gene Therapy, 2023, 30(1):18-30.
8. Wang Q H, Wang J, Ling Z P, et al. Phase I clinical trial of intracerebral injection of lentiviral-ABCD1 for the treatment of cerebral adrenoleukodystrophy[J]. Science Bulletin, 2024, 69(16):2596-2603.